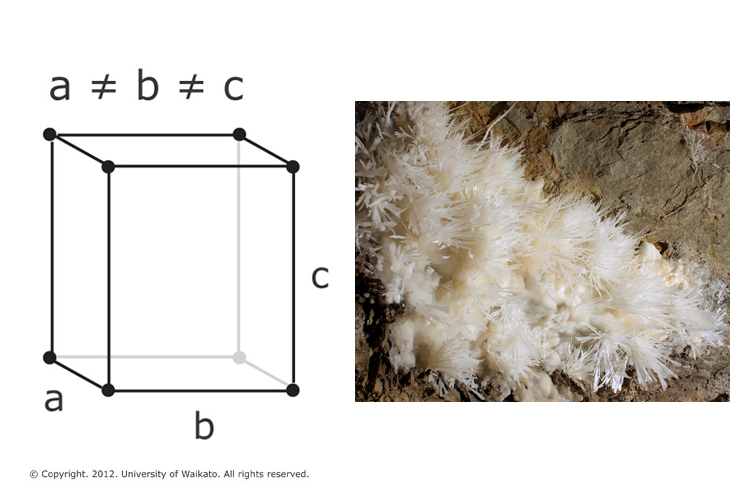
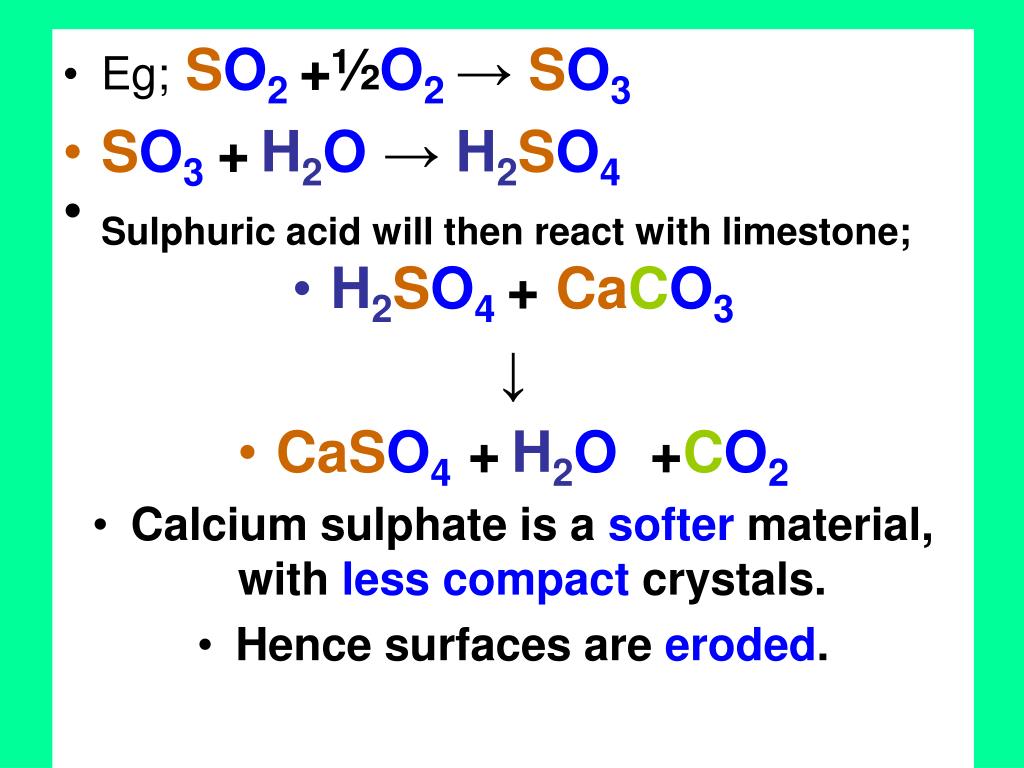
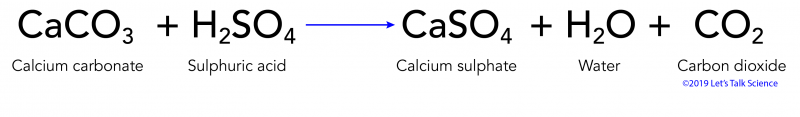
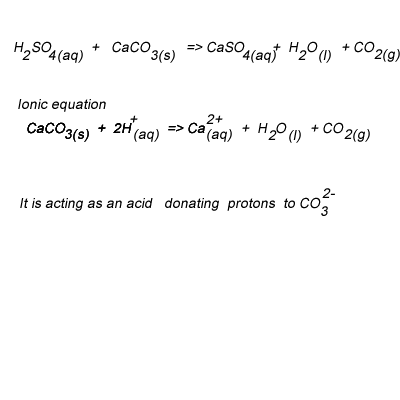Consumption of CO 2 and production of HCO 3 − in silicates and calcium... | Download Scientific Diagram
What happens when sulphuric acid reacts with calcium carbonate? What is the chemical equation of the reaction? How do you identify the gas evolved? - Quora

28.5 g of calcium carbonate (CaCOn reacts with an excess of sulfuric acid (H SOg) to farm calcium sulfate - brainly.com

Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution

reactivity - How could mass increase when sulfuric acid is added to calcium carbonate? - Chemistry Stack Exchange

25 gram of calcium carbonate completely reacts with sulphuric acid to produce 11g of carbon dioxide. the - Brainly.in






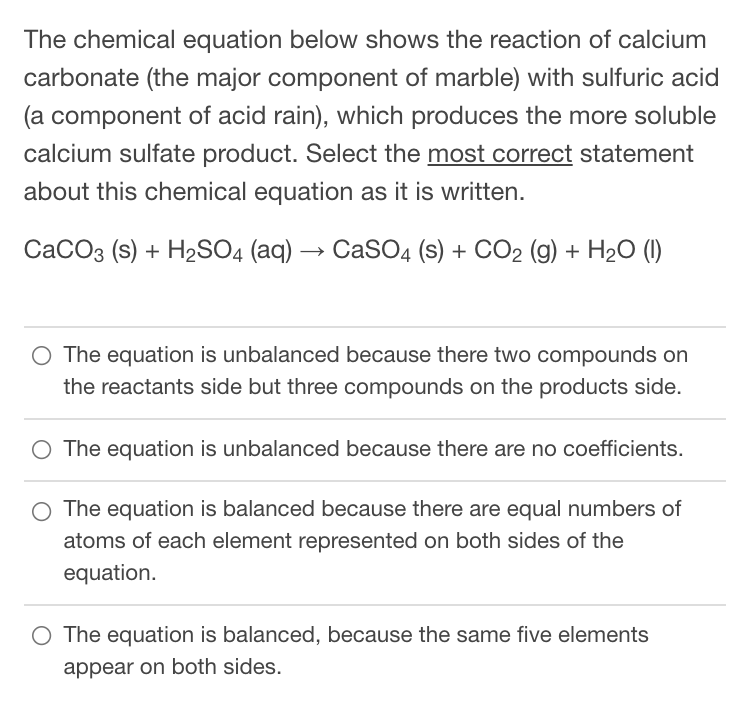


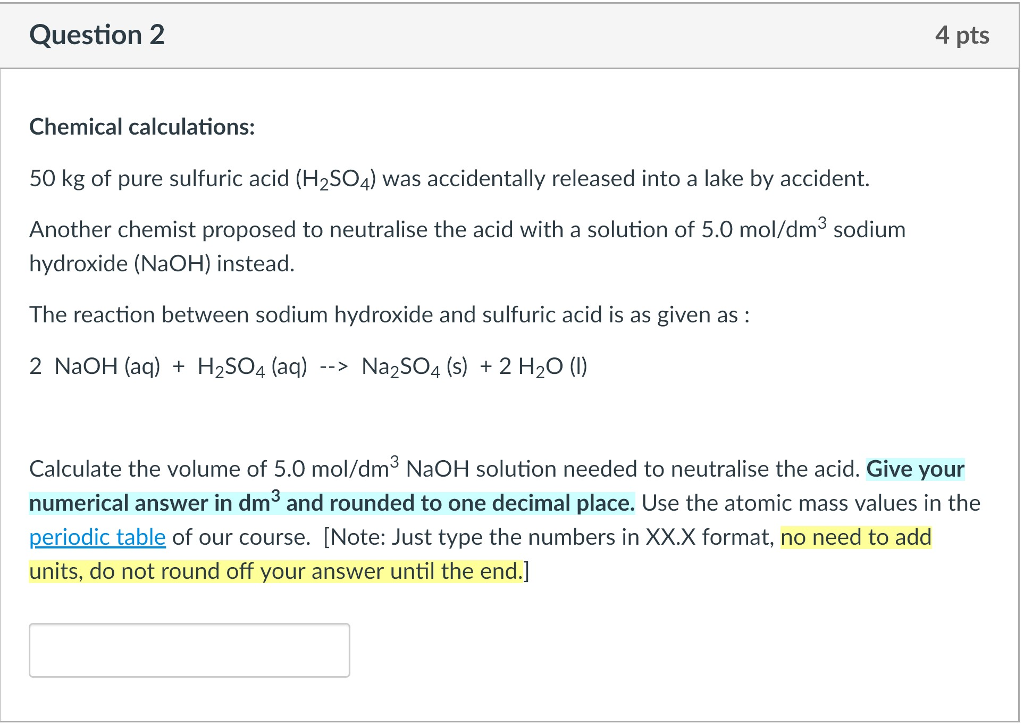

![Solved Question 1 [15] During a production process, a waste | Chegg.com Solved Question 1 [15] During a production process, a waste | Chegg.com](https://media.cheggcdn.com/study/bf1/bf1b7069-44ef-41be-856e-c8a738df0f9c/image)